Mac-Man unchained: A new strategy against Graft-versus-Host Disease
This week, a new paper appeared in Blood, where we report a crucial discovery in understanding graft-versus-host disease (GvHD), the most severe complication after stem cell transplantation. We show how harmful donor T cells exploit a molecular “don’t eat me” signal to evade clearance by the body’s immune defenses — a finding that not only explains a central disease mechanism but also opens new avenues for targeted therapies.
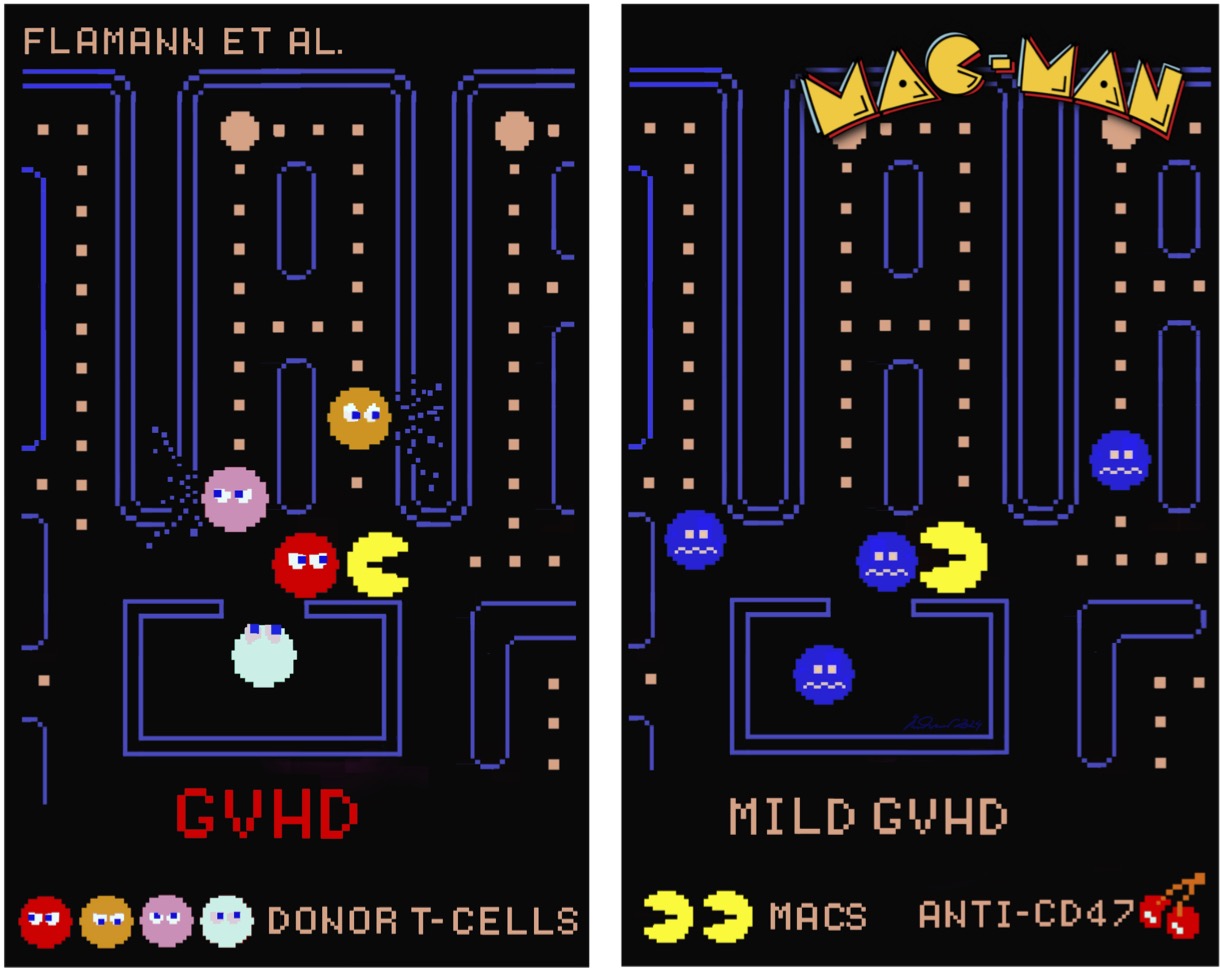
“Mac-Man” macrophages regain their ability to clear harmful CD47-overexpressing T cells – abundant in patients and animals with GvHD – when anti-CD47 antibodies are administered, mitigating disease. Illustration by Andreas Beilhack, inspired by the digital art of Toru Iwatani, the original creator of Pac-Man.
In this joint work, Cindy Flamann and Haroon Shaikh together with researchers from the team of Heiko Bruns at University Hospital Erlangen and the Beilhack lab at University Hospital Würzburg investigated why the body’s natural defense mechanisms fail in GvHD. Normally, macrophages – among lab scientists sometimes nicknamed the “Mac-Men” of the immune system in reference to the arcade game Pac-Man – patrol the body to eliminate harmful or overactive cells. Our new study shows that in GvHD, donor T cells display unusually high levels of the surface protein CD47. Acting as a “don’t eat me” signal, CD47 prevents macrophages from recognizing and engulfing these pathogenic T cells. As a result, the T cells accumulate in tissues such as the intestine, where they trigger severe inflammation.
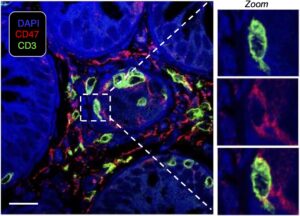
Alloreactive T cells upregulate CD47, shielding them from phagocytosis (“being eaten”) by macrophages. Shown here are alloreactive T cells from a patient biopsy infiltrating the intestinal tract and driving acute graft-versus-host disease (GvHD). Flamann & Shaikh et al., Blood 2025.
To uncover this mechanism, the team combined patient biopsies, high-resolution cellular analyses, and experimental models. They found that activation of the T-cell receptor drives CD47 expression through NF-κB signaling, effectively cloaking the T cells from macrophage clearance. Importantly, when donor T cells lacking CD47 were transplanted into mice, macrophages could once again remove the harmful cells. This led to reduced disease severity and improved survival. Likewise, treatment with anti-CD47 antibodies restored macrophage activity, protected intestinal tissues, and dampened inflammation.
These findings have significant therapeutic implications. Since CD47 is also frequently overexpressed on leukemia cells, therapies targeting this pathway could achieve two goals at once: controlling GvHD while simultaneously reinforcing the beneficial graft-versus-leukemia effect. With several anti-CD47 antibodies already in clinical development, translation of this strategy into patient care is within reach.
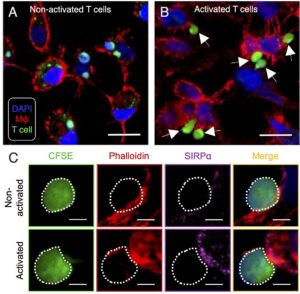
Macrophages can engulf donor T cells (A). However, when activated, donor T cells upregulate CD47, allowing them to cling to but escape macrophages (B). Blocking CD47 restores clearance of these pathogenic T cells (C). Flamann & Shaikh et al., Blood 2025.
This study exemplifies the power of collaborative research. It was conducted by the Heiko Bruns Lab at the University Hospital Erlangen and the Beilhack Lab at the University Hospital Würzburg within the DFG TRR221 GvH-GvL consortium Erlangen–Regensburg–Würzburg. Of particular note, Dr. Haroon Shaikh (Würzburg) and doctoral researcher Cindy Flamann (Erlangen) contributed equally as first authors, driving the experimental work and analyses that made these discoveries possible.
“Our findings uncover a mechanism that helps explain why GVHD-causing T cells escape immune regulation and show that this vulnerability can be therapeutically targeted,” said PD Dr. Heiko Bruns. “This interdisciplinary collaboration highlights how basic immunology can drive new strategies for the clinic,” added Prof. Andreas Mackensen and Prof. Andreas Beilhack, emphasizing the strength of the TRR221 GvH-GvL consortium.
Reference:
Flamann C*, Shaikh H*, Matos C, Kreutz M, Ali H, Kern MAG, Büttner-Herold M, Jacobs B, Völkl S, Lischer C, Kellner C, Berges J, Bitterer K, Saul D, Goel M, Link-Rachner CS, Zernecke A, Weber D, Mougiakakos D, Mackensen A, Beilhack A, Bruns H. (2025). Augmented CD47 expression impairs alloreactive T-cell clearance after allo-HCT. Blood 146(11):1359-1373.
